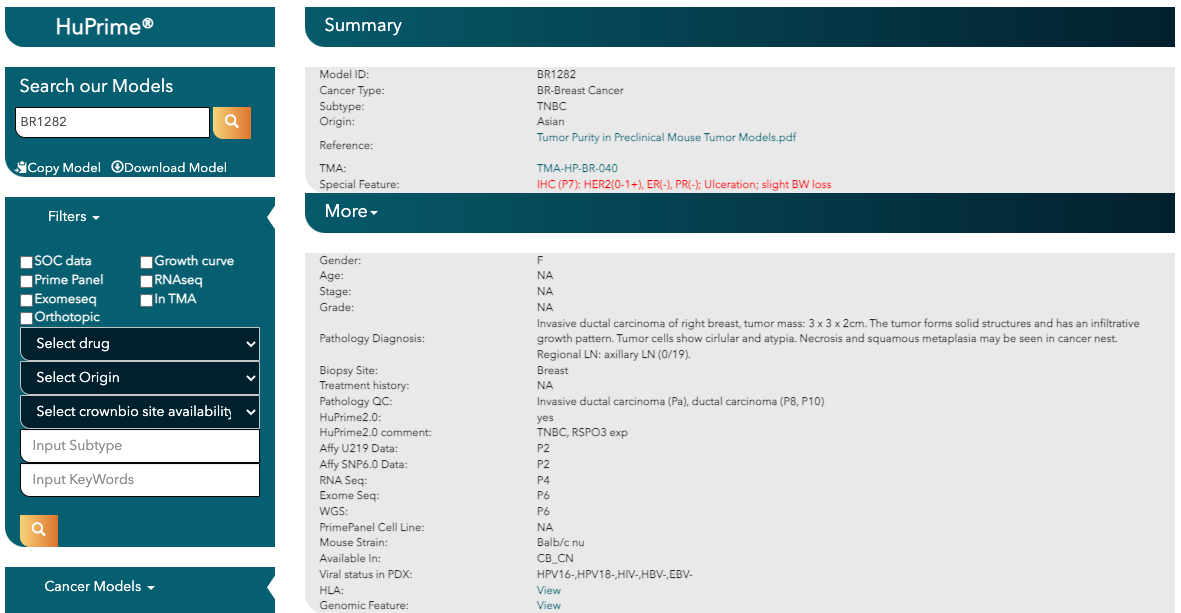
November 21, 2024
- Our Services
- Platforms
- Target Solutions
- Technologies
- Service Types
- Our Science
- About Us
- Contact us
November 21, 2024
Patient-derived xenografts - PDX offer the most translational preclinical model for efficacy screening in cancer drug development. Derived directly from patient tumors and never adapted to grow in vitro, PDX models reflect the heterogeneity and diversity of the human patient population.
PDX give you an accurate, predictive model of how your treatment will perform, well before entering into expensive clinical trials.
Browse PDX Models By Indication
Extensive characterization data in our searchable PDX model database, HuBase™, include: RNAseq of over 1,500 models, whole exome sequencing of over 680 models, histology, growth kinetics, and SoC and investigative treatment data.
Evaluate blood cancer therapeutic candidates with validated, stable, truly patient representative leukemia models.
HuKemia is Crown Bioscience’s collection of validated blood cancer PDX models, fully annotated with patient information, diagnosis, and clinical treatments. These models have been fully quality controlled by our pathologists and are available with genotyping and phenotyping data.
Crown Bioscience’s validated and well-characterized HuKemia models provide many advantages:

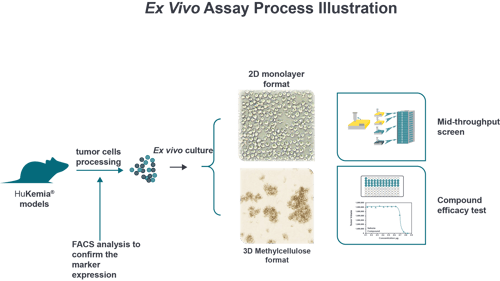
PDX models for blood cancer are historically very challenging to produce, due to their low take rate in vivo. We overcame these challenges to develop a unique collection of AML and ALL PDX models to better support your preclinical research.
Derived directly from patient bone marrow aspirate, our PDX models better reflect the heterogeneity of leukemia. The models present as stable disease and are transferable through passage. This provides highly reproducible results and availability for repeated studies.
Stable models with typical leukemia symptoms and eventual mortality, truly representative of the human condition.Progress targeted agents by choosing PDX models with patient-relevant mutationse.g. IDH2 mutation, FLT3-ITD(+), BCR/ABL(+).Explore extensive model charcterization for pathology, growth characteristics, and response to standard of care and experimental agents.Search for models to meet you research needs through genetic and genomic annotations. Models are charcterized for gene expression, gene copy number, mutations, and fusions.
Log in to explore Crown Bioscience's well-characterized Patient-Derived Xenograft's through our searchable database. Access genomic, molecular, and phenotypic data including tumor growth curves, standard of care response, histopathology data, and patient information on the worlds largest collection
Browse or search our collection to access patient information and model specific characteristics.
Search PDX genomic information e.g. RNAseq gene expressions data and WES gene copy number data for MET amplification.
PDX Mouse Clinical Trials offer a unique advantage for oncology drug developers by providing:

Figure. A representative PDX Mouse Clinical Trial Design
For oncology drug discovery teams, Crown Bioscience offers unmatched expertise in PDX mouse clinical trials (MCTs) to accelerate clinical translation and reduce attrition rates.
Crown Bioscience offers end-to-end support, replicating clinical trial conditions preclinically to enhance success rates, reduce costs, and speed up drug development. With 12+ years of experience and 300+ successful MCT projects, we are your trusted partner from preclinical to clinical stages.
© 2024 Crown Bioscience. All Rights Reserved.


© 2024 Crown Bioscience. All Rights Reserved. Privacy Policy
2024-09-25
2023-07-21
site_page
Integrated Solutions - MCT