- Our Services
- Platforms
- Target Solutions
- Technologies
- Service Types
- Our Science
- About Us
- Contact us
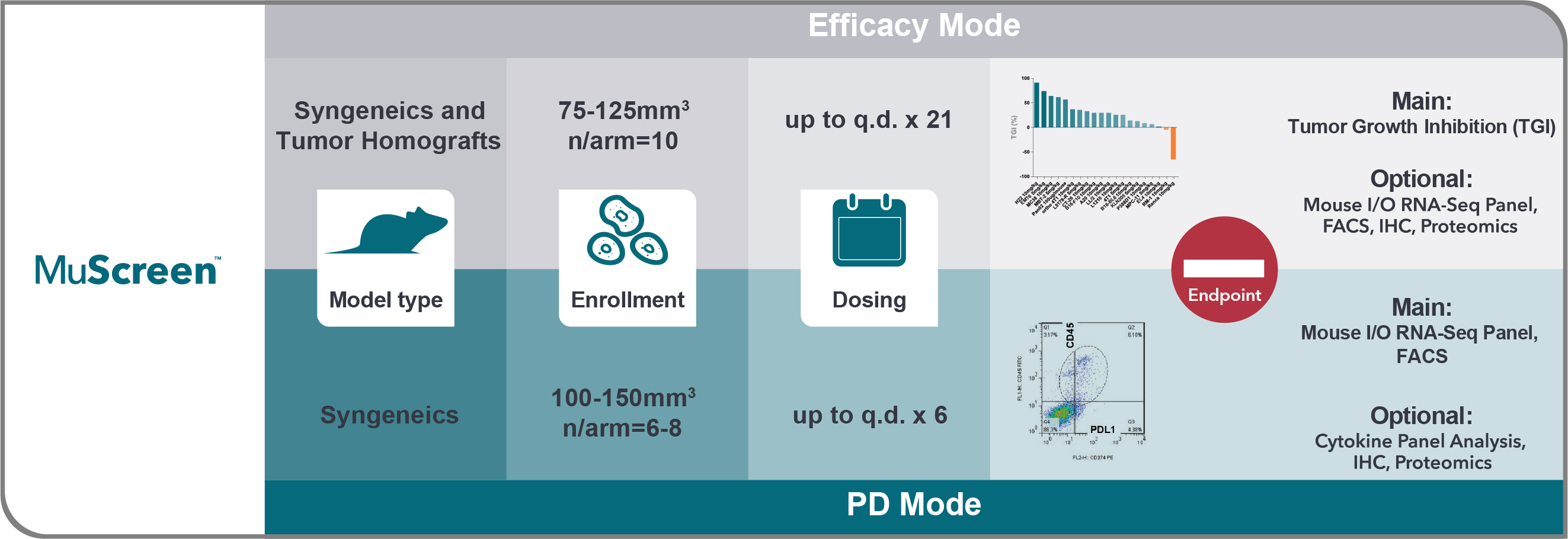
MuScreen is the first high throughput in vivo screen for evaluating immunotherapy and immune modulating compounds across both well-characterized syngeneic and unique tumor homograft models.
Agent efficacy is evaluated using syngeneic and/or tumor homograft model panels, with PD effects assessed using syngeneic models only. MuScreen is a cost-effective screening platform, with a free vehicle arm and shared cost of the positive control arm.
| Model Type | Model Features |
Efficacy | PD | ||
|---|---|---|---|---|---|
|
US
|
China
|
US
|
China | ||
| Syngeneic | Display immune heterogeneity and diversity observed in the clinic |
6 models |
6 models |
12 models |
|
| Tumor Homograft | Transplants of GEMM tumors into a syngeneic host, preserving original GEMM tumor molecular and histopathology and clinically relevant oncogenic driver mutations |
6 models |
|||
12 Model Efficacy and PD ModeStudy Site: Crown Bioscience China
|
6 Model Syngeneic Efficacy and PD ModeStudy Site: Crown Bioscience San Diego
|
|
Registration Deadline
|
Study Initiation
|
|---|---|
|
January 12th, 2024
|
March 1st, 2024
|
|
May 10th, 2024
|
June 21st, 2024
|
|
September 6th, 2024
|
October 18th, 2024
|
Your privacy is important to us.
We'll never share your information.
© 2025 Crown Bioscience. All Rights Reserved.
Privacy Policy | Imprint | Terms of Service | Privacy Preferences


© 2025 Crown Bioscience. All Rights Reserved. Privacy Policy
2024-10-15
2021-12-20
landing_page
Integrated Solutions - In Vitro - I/O Targets/Combinations