- Our Services
- Platforms
- Target Solutions
- Technologies
- Service Types
- Our Science
- About Us
- Contact us

Study unique immune-related adverse events (irAEs) associated with immunotherapies, using HuGEMM™ and HuCELL™ human target knock-in models. The platforms feature humanized drug targets within a fully functional murine immune system, providing an ideal method for assessing the complex nature of on-target and off-target toxicities of human-specific immunotherapies.
Efficacy and toxicity of human-specific therapies can be assessed in the same animal, enabling determination of the optimal therapeutic window in the same species and providing quick, cost-effective, and robust data to guide future drug development work.
HuGEMM models are immunocompetent chimeric mouse models, engineered to express humanized drug targets (instead of their murine counterparts) such as genes encoding for immune checkpoint proteins.
| Single Knock-in | Double Knock-in | Triple Knock-in |
|---|---|---|
| B7H3 | TIGIT/PVR | CD47/Sirpα/PD-1 |
| BTLA | PD-L1/TIM-3 | CD47/Sirpα/PD-L1 |
| CCR2 | PD-L1/TIGIT | PD-1/PD-L1/CD137 |
| CCR8 | PD-L1/OX40 | PD-1/PD-L1/CTLA4 |
| CD137 | PD-L1/LAG3 | PD-1/PD-L1/IDO-1 |
| CD27 | PD-L1/CTLA4 | PD-1/PD-L1/LAG-3 |
| CD28 | PD-L1/CD47 | PD-1/PD-L1/OX40 |
| CD38 | PD-L1/CD40 | PD-1/PD-L1/TIGIT |
| CD39 | PD-L1/CD27 | PD-1/PD-L1/TIM-3 |
| CD3E | PD-L1/CD137 | PD-1/TIM-3/TIGIT |
| CD40 | PD-1/Tim3 | |
| CD47 | PD-1/TIGIT | |
| CD73 | PD-1/Sirpα | |
| CTLA4 | PD-1/PD-L1 | |
| GITR | PD-1/OX40 | |
| IL-1b | PD-1/LAG3 | |
| LAG3 | PD-1/GITR | |
| OX40 | PD-1/CTLA4 | |
| OX40L | PD-1/CD47 | |
| PD-1 | PD-1/CD40 | |
| PD-L1 | PD-1/CD28 | |
| SIGLEC15 | PD-1/CD27 | |
| Sirpα | PD-1/CD137 | |
| STING | PD-1/BTLA | |
| TIGIT | OX40/CD137 | |
| TIM-3 | NKG2A/CD94 | |
| TNFR2 | IL2RA/IL2 | |
| VEGFR2 | CTLA4/Tim3 | |
| CTLA4/OX40 | ||
| CTLA4/LAG3 | ||
| CTLA4/CD137 | ||
| CD47/Sirpα | ||
| CD40/CD137 | ||
| CD27/CD137 |
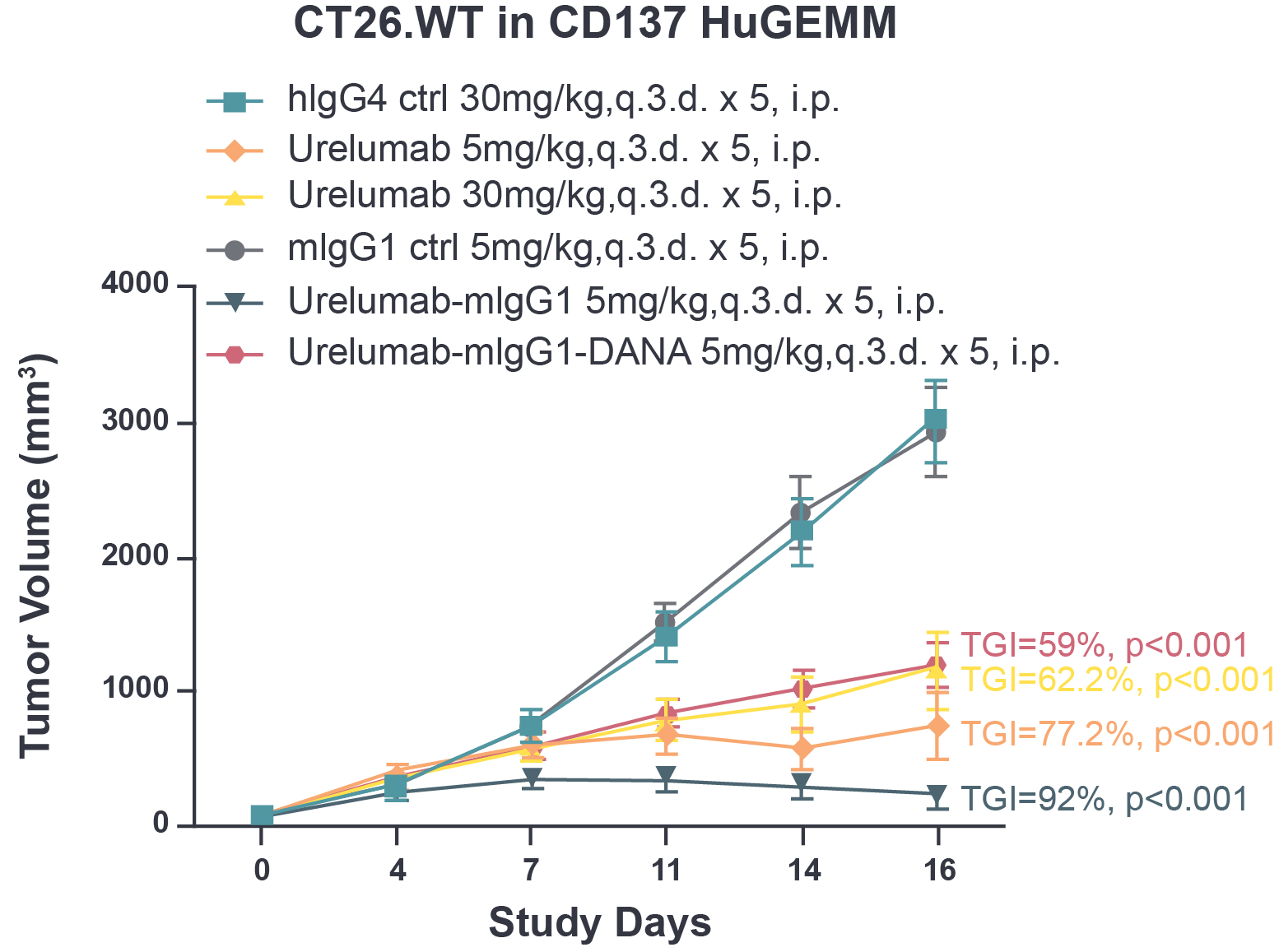
Fig 1. The tumor growth inhibition of urelumab and chimeric urelumab on murine colon tumor CT26.WT in CD137 HuGEMM model. Tumor growth inhibition (TGI) was calculated as: TGI% = (1-Ti/Vi)*100; Ti as the mean tumor volume of the treatment group on the measurement day; Vi as the mean tumor volume of control group at the measurement day.
Fig 2. Measurement of liver toxicity based on ALT (alanine aminotransferase) and AST (aspartate aminotransferase) levels in CD137 HuGEMM animals. Fasting serum level of ALT (alanine aminotransferase) and AST (aspartate aminotransferase) were measured 2 and 7 days after final dose. One way ANOVA *, **, and *** refer to p<0.05, p<0.01, and p<0.001, respectively.
Fig 3. Histological assessment of liver inflammation following urelumab and chimeric urelumab treatment in CD137 HuGEMM in vivo model. H&E staining of liver tissue. Liver inflammation was evaluated on a scale of 0-3: 0 = none; 1 = mild; 2 = moderate; 3 = severe.
Fig 4. Infiltration of CD45+ immune cells in the liver following urelumab and chimeric urelumab treatment in CD137 HuGEMM in vivo model, as indicated by IHC staining - CD45+ cell density were measured by HALO v3.0.311.363.
Tell us about your needs
© 2025 Crown Bioscience. All Rights Reserved.


© 2025 Crown Bioscience. All Rights Reserved. Privacy Policy
2024-04-23
2021-10-23
site_page