Close
Contact UsLanguageSearch
- Our Services
- Platforms
- In Vivo
- PDX Models
- CDX Models
- Syngeneic Models
- Humanized Models
- Humanized PBMC Models
- Humanized Genetically Modified Mouse Models
- Tumor Homograft Models
- Orthotopics with Advanced Imaging
- In Vitro
- 2D Cell Lines and Panels
- Syngeneic Cell Lines
- CRISPR/Cas9
- Organoids
- High Content Imaging
- Tumor Tissue Microarray
- Biospecimen Solutions (Human) BackExit
- Biomarkers and Bioanalysis
- Data Science and Bioinformatics
- In Vivo
- Target Solutions
- 3D Bone Marrow Models
- ADCs
- AML Drug Development
- EGFR
- Drug Resistance
- Immuno-Oncology
- KRAS
- Patient Tissue
- Technologies
- Bioinformatics
- Preclinical Biomarker Discovery
- Genomics Data Analysis
- Proteomics Data Analysis
- Mouse I/O RNA-Seq Panel
- Cell Line Authentication with Deep Sequencing
- CrownSyn™
- Genomics
- Standard Genomics Services
- Next Generation Sequencing Services
- Cell Line Authentication (CLA) with Deep Sequencing
- Mouse I/O RNA-Seq Panel
- NGS Data Analysis
- Biomarker Analysis
- Biofluid Testing
- Cytokine & Chemokine Profiling
- Flow Cytometry
- Rare Cell Analysis
- Mass Spectrometry-based Proteomics
- In Vitro High Content Imaging BackExit
- Mass Spectrometry-based Proteomics
- Ex Vivo Patient Tissue
- Spatial Multi-Omics Analysis
- CRISPR/Cas9
- Bioinformatics
- Service Types
- Laboratory Services
- Genomics and Transcriptomics
- Proteomics Services
- Spatial Biology and Digital Pathology
- Immune Monitoring
- Bioanalytical Services
- Biomarker Discovery
- PDX Mouse Clinical Trial
- Biomarkers of Response
- Biomarker Discovery and Companion Diagnostics
- Signatures of Response: Predictive Biomarkers
- Pharmacology & Bioanalytical Services
- Biofluid Testing
- Rodent Immuno-Safety Models
- Safety Pharmacology Studies
- Structural Biology
- Target Validation
- Screens BackExit
- Toxicology
- DMPK Services
- Consulting
- Efficacy Testing
- Laboratory Services
- Platforms
- Our Science BackExit
- About Us
- Our Company
- Our Purpose
- Our Responsibility
- Leadership Team
- Scientific Advisory Board
- News & Events
- Awards & Testimonials
- Career Opportunities
- Contact us


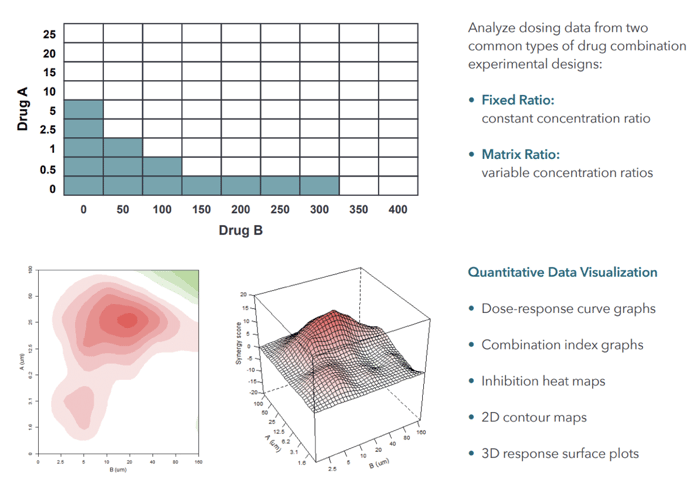
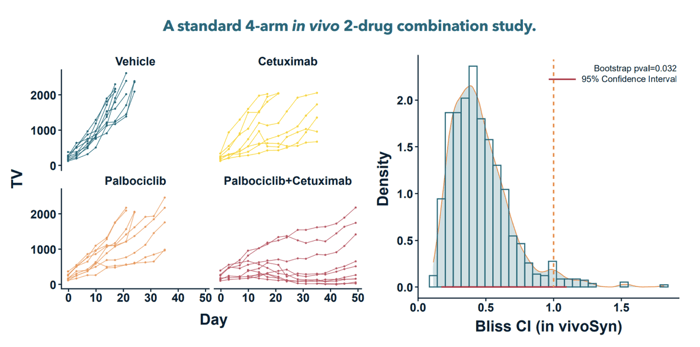
 Cancer is not a singular disease but a myriad of ailments with complex gene and molecular interactions. While a single drug might target one specific pathway, cancer can adapt by leveraging other untargeted pathways, leading to drug resistance. This brings drug combinations into the limelight, which can offer multiple advantages:
Cancer is not a singular disease but a myriad of ailments with complex gene and molecular interactions. While a single drug might target one specific pathway, cancer can adapt by leveraging other untargeted pathways, leading to drug resistance. This brings drug combinations into the limelight, which can offer multiple advantages: