Close
Contact UsLanguageSearch
- Our Services
- Platforms
- In Vivo
- PDX Models
- CDX Models
- Syngeneic Models
- Humanized Models
- Humanized PBMC Models
- Humanized Genetically Modified Mouse Models
- Tumor Homograft Models
- Orthotopics with Advanced Imaging
- In Vitro
- 2D Cell Lines and Panels
- Syngeneic Cell Lines
- CRISPR/Cas9
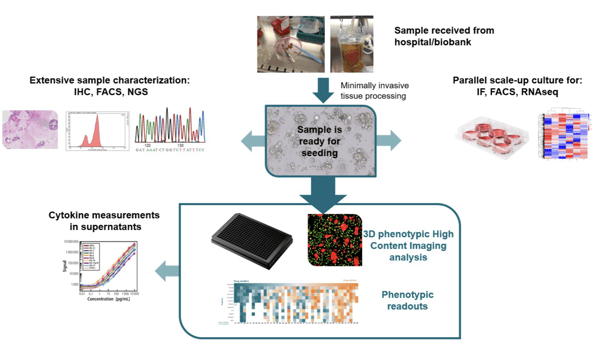
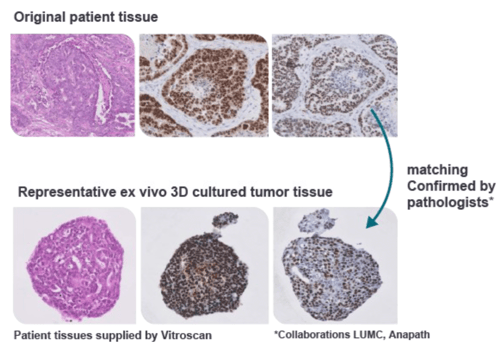
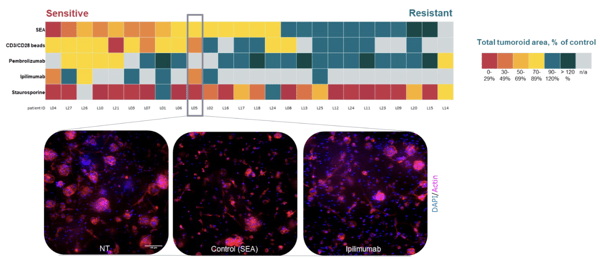
- Organoids
- High Content Imaging
- Tumor Tissue Microarray
- Biospecimen Solutions (Human) BackExit
- Biomarkers and Bioanalysis
- Data Science and Bioinformatics
- In Vivo
- Target Solutions BackExit
- Technologies
- Bioinformatics
- Preclinical Biomarker Discovery
- Genomics Data Analysis
- Proteomics Data Analysis
- Mouse I/O RNA-Seq Panel
- Cell Line Authentication with Deep Sequencing
- CrownSyn™
- Genomics
- Standard Genomics Services
- Next Generation Sequencing Services
- Cell Line Authentication (CLA) with Deep Sequencing
- Mouse I/O RNA-Seq Panel
- NGS Data Analysis
- Biomarker Analysis
- Biofluid Testing
- Cytokine & Chemokine Profiling
- Flow Cytometry
- Rare Cell Analysis
- Mass Spectrometry-based Proteomics
- In Vitro High Content Imaging BackExit
- Mass Spectrometry-based Proteomics
- Ex Vivo Patient Tissue
- Spatial Multi-Omics Analysis
- CRISPR/Cas9
- Bioinformatics
- Service Types
- Laboratory Services
- Genomics and Transcriptomics
- Proteomics Services
- Spatial Biology and Digital Pathology
- Immune Monitoring
- Bioanalytical Services
- Biomarker Discovery
- PDX Mouse Clinical Trial
- Biomarkers of Response
- Biomarker Discovery and Companion Diagnostics
- Signatures of Response: Predictive Biomarkers
- Pharmacology & Bioanalytical Services
- Biofluid Testing
- Rodent Immuno-Safety Models
- Safety Pharmacology Studies
- Structural Biology
- Target Validation
- Screens BackExit
- Toxicology
- DMPK Services
- Consulting
- Efficacy Testing
- Laboratory Services
- Platforms
- Our Science BackExit
- About Us
- Our Company
- Our Purpose
- Our Responsibility
- Leadership Team
- Scientific Advisory Board
- News & Events
- Awards & Testimonials
- Career Opportunities
- Contact us